Background: The anti-apoptotic protein Mcl-1 contributes to the pathophysiology of certain B-cell malignancies and AML, and dependence on Mcl-1 is associated with resistance to venetoclax. Efforts to target Mcl-1 through direct inhibition have faced challenges due to cardiovascular toxicities. Voruciclib, a potent oral CDK9 inhibitor (CDK9i), was shown to indirectly downregulate Mcl-1 protein levels and synergize with venetoclax in preclinical models in lymphoid and myeloid malignancies. In solid tumors, voruciclib maximum tolerated dose (MTD) was defined as 350 mg with continuous daily dosing, and 600 mg when used on days 1-14 on a 21-day cycle. This phase 1 study is the first to evaluate the safety, dose-limiting toxicities (DLT), preliminary efficacy, pharmacokinetics, and pharmacodynamics of voruciclib in R/R hematologic malignancies (NCT03547115).
Methods: Patients (pts) with relapsed B-cell NHL, CLL, or AML aged ≥18 years who had ECOG performance status ≤1, disease progression after standard therapies, adequate hepatic and renal function, and no prior CDK9i were eligible. The study followed a 3+3 design and DLTs were assessed in Cycle 1. Voruciclib was administered daily continuously at a starting dose of 50 mg on a 28-day cycle (Cohort I). After 2 DLTs were observed at 100 mg in AML pts, dosing was changed to daily on days 1-14 on a 28-day cycle (Cohort II). Also, pts with prior allogeneic transplant were excluded and dose escalation (100, 150, 200 mg) proceeded separately in AML and B-cell malignancies. Disease response was assessed by investigators using the Lugano, 2008 iwCLL, and 2017 ELN criteria.
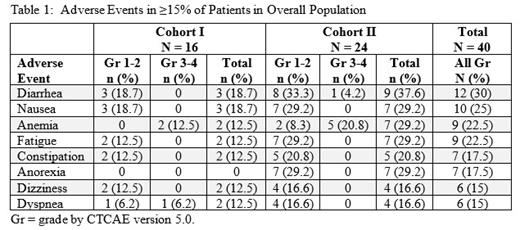
Results: 40 pts were enrolled: 21 AML, 19 NHL (9 DLBCL, 3 CLL, 3 MCL, 3 FL and 1 MZL). Cohort I enrolled 16 pts, 8 each at 50 mg and 100 mg. Cohort II enrolled 24 pts (17 AML and 7 B-cell malignancies), including 6 at 100 mg, 7 at 150 mg, and 11 at 200 mg. Dose escalation in Cohort II did not reach the MTD and was stopped at 200 mg, a dose that achieved plasma concentrations sufficient for target inhibition. Median age was 70 years (range 40-90), 72.5% of pts were ≥65 years, and 77.5% had ECOG performance status = 1. Pts had a median of 3 prior therapies (range, 1-8). Median duration on drug was 5 weeks (range 1-22). Drug-related serious adverse events (AEs) were reported in Cohort I only. Two pts with AML had a DLT of Grade (Gr) 3 interstitial pneumonitis at 100 mg; both had had allogeneic transplant and active graft versus host disease, one of whom also had a concurrent Gr 3 differentiation syndrome. One pt with DLBCL at 50 mg had Gr 3 hypoxemic respiratory failure in Cycle 4. In Cohort II, no DLTs were observed and there were no non-hematologic Gr 3-5 drug-related AEs. In the overall population, the most common AEs were diarrhea (30%), nausea (25%), anemia (22.5%), fatigue (22.5%), constipation (17.5%), dizziness (15%) and dyspnea (15%) [Table 1). One pt with AML had Gr 3 hyperbilirubinemia. There was no evidence of drug-related neutropenia in pts with NHL. No significant cardiovascular AEs were observed. One pt with AML in Cohort I achieved a morphologic leukemia-free state. At 200 mg, 5 of 10 AML pts (50%) had stable disease. Voruciclib pharmacokinetics was dose proportional, mean accumulation ratio was 2.4, mean T max was 4 hours, and at 200 mg, steady state C max was 925 ng/mL and mean C trough was 442 ng/mL (~1 microM). Analysis of Mcl-1 protein expression in normal lymphocytes by flow cytometry showed a trend of decrease in on-treatment samples compared to pre-treatment. Consistent with this, RNA-Seq analysis of 3 paired CLL samples confirmed rapid (after 6 hours) downregulation of Mcl-1 mRNA transcript in the malignant B-cells. Furthermore, scRNA-Seq analysis of 2 longitudinal AML samples demonstrated downregulation of MYC transcriptional program and oxidative phosphorylation in circulating AML blasts, both evident 24 hours after start of voruciclib and ongoing at C1D15.
Conclusions: Voruciclib at doses up to 200 mg administered on 14 consecutive days on a 28-day cycle was well tolerated, with no DLTs observed, and no cardiovascular toxicities. There was no significant myelosuppression in pts with B-cell malignancies. As the safety profile suggested non-overlapping toxicities with venetoclax, the study is now evaluating voruciclib in combination with venetoclax in pts with R/R AML to exploit dual inhibition of Bcl-2 and Mcl-1.
Disclosures
Davids:BMS: Consultancy; BeiGene: Consultancy; Janssen: Consultancy; Merck: Consultancy; Mingsight Pharmaceuticals: Consultancy; Eli Lilly: Consultancy; Genentech: Consultancy, Research Funding; Curio Science: Consultancy; Aptitude Health: Consultancy; Research to Practice: Consultancy; Surface Oncology: Research Funding; ONO Pharmaceuticals: Consultancy; MEI Pharma: Research Funding; Takeda: Consultancy; Novartis: Research Funding; Secura Bio: Consultancy; TG Therapeutics: Consultancy, Research Funding; Adaptive Biosciences: Consultancy; Ascentage Pharma: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding. Alvarado Valero:CytomX Therapeutics: Consultancy; Jazz: Research Funding; BerGenBio: Research Funding; MEI Pharma: Research Funding; Astex: Research Funding; Sun Pharma: Consultancy, Research Funding; FibroGen: Research Funding; Daiichi-Sankyo: Research Funding. Diefenbach:Gillead: Current equity holder in publicly-traded company; OverT Therapeutics: Current equity holder in private company; Genmab. Abbvie, Regeneron, F. Hoffmann-La Roche Ltd / Genentech, Inc., Seattle Genetics, Merck: Membership on an entity's Board of Directors or advisory committees; F. Hoffmann-La Roche Ltd / Genentech, Inc., BMS, Merck, Abbvie, Novartis, Celgene, Cargo, Nekktar: Research Funding. Dinner:Novartis: Research Funding; BMS: Research Funding; Pfizer: Research Funding; Rigel: Research Funding; Kite/Gilead: Research Funding. Al Malki:Hasna Biopharma: Membership on an entity's Board of Directors or advisory committees; CareDx: Consultancy, Membership on an entity's Board of Directors or advisory committees; NMDP: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding; NexImmune: Consultancy, Research Funding; T scan: Consultancy; Stemline Therapeutics: Membership on an entity's Board of Directors or advisory committees. Begna:Novartis: Membership on an entity's Board of Directors or advisory committees; MEI Pharma: Research Funding; Immunogen: Research Funding. Kallam:Abbvie: Consultancy. Abedin:Incyte: Research Funding; AltruBio: Research Funding; AbbVie: Consultancy, Honoraria; Actinium Pharmaceutical: Research Funding; Daichii Sankyo: Consultancy, Honoraria; Servier: Consultancy, Honoraria. Miao:MEI Pharma: Consultancy, Ended employment in the past 24 months. Ghalie:MEI Pharma: Current Employment, Current equity holder in publicly-traded company. Danilov:Bayer: Research Funding; Nurix: Consultancy, Research Funding; Lilly Oncology: Consultancy, Research Funding; MEI: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Cyclacel: Research Funding; Janssen: Consultancy; Astra Zeneca: Consultancy, Research Funding; Bristol Meyers Squibb: Consultancy, Research Funding; Genentech: Consultancy; GenMab: Consultancy, Research Funding; Merck: Consultancy; Beigene: Consultancy, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal